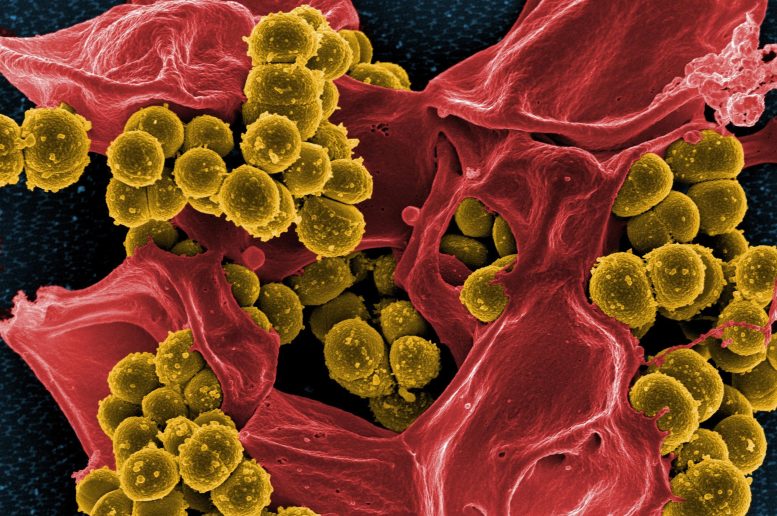
Worldwide, many strains of the bacterium Staphylococus aureus are already resistant to all antibiotics except vancomycin. But as bacteria are becoming resistant to this once powerful antidote, S. aureus has moved one step closer to becoming an unstoppable killer. Now, researchers at the University of North Carolina at Chapel Hill have not only identified the mechanism by which vancomycin resistance spreads from one bacterium to the next, but also have suggested ways to potentially stop the transfer. The work, led by Matthew Redinbo, professor of chemistry at UNC’s College of Arts and Sciences, addresses the looming threat of incurable staph infections – a global public health problem that has mobilized scientists across disciplines to work together to identify the Achilles heel of these antibiotic-resistant bacteria. “We used to live in a world where antibiotics could readily cure bacterial disease,” said Redinbo. “But this is clearly no longer the case. We need to understand how bacteria obtain resistance to drugs like vancomycin, which served for decades as the ‘antibiotic of last resort.’” In his work, Redinbo and his team targeted a bacterial enzyme known as Nicking Enzyme in Staphylococcus, or NES. The enzyme has long been known to interact with plasmids, circular pieces of double-stranded DNA within bacteria that are physically separate from the bacterial chromosome. Plasmids commonly contain antibiotic-resistance genes, and can make the machinery necessary to transfer these genes from an infected bacterium to an uninfected one. By revealing the crystal structure of NES, the researchers found that this enzyme nicks one strand of the plasmid at a very specific site—and in a very specific way. It turns out that NES forms two loops that work together to pinch one strand of the plasmid at a particular groove in the DNA to cut it. This strand is now free to leave its host and transfer to a nearby bacterium, making them resistant to vancomycin. Moreover, Redinbo was able to capture a snapshot of the enzyme bound to the plasmid. “As a structural biologist, it’s all about the pictures for me,” said Redinbo. “And it was this picture that confirmed the precise location on which NES works.” With this information, Redinbo knew the groove on the DNA that the enzyme recognize and could design a small synthetic molecule that would sit on this groove and block NES. Teaming up with colleagues at the California Institute of Technology, Redinbo did just that. The molecule prevented NES from nicking the DNA, which could prevent the resistance genes from spreading. According to Redinbo and colleagues, this small synthetic molecule could help guide future research aimed at developing effective therapies for strains of antibiotic-resistant S. aureus. “This is really exciting for us,” said Redinbo, who is also a professor at UNC’s School of Medicine and a member of the Lineberger Comprehensive Cancer Center. “It opens the door for potentially stopping the spread of antibiotic resistance—and that’s exactly what we need in this post-antibiotic era.” The work was published this week in the online early edition of the Proceedings of the National Academy of Sciences. Reference: “Molecular basis of antibiotic multiresistance transfer in Staphylococcus aureus” by Jonathan S. Edwards, Laurie Betts, Monica L. Frazier, Rebecca M. Pollet, Stephen M. Kwong, William G. Walton, W. Keith Ballentine, III, Julianne J. Huang, Sohrab Habibi, Mark Del Campo, Jordan L. Meier, Peter. B. Dervan, Neville Firth and Matthew R. Redinbo, 28 January 2013, Proceedings of the National Academy of Sciences.DOI: 10.1073/pnas.1219701110